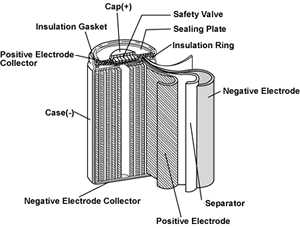
A typical cell construction is cylindrical for greatest energy density
Click to go large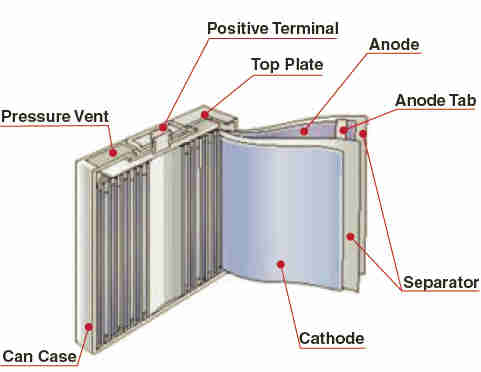
However prismatic (rectangular) cells are more popular nowadays for packaging reasons

A typical cell construction is cylindrical for greatest energy density
Click to go large
However prismatic (rectangular) cells are more popular nowadays for packaging reasons
Two Way Radio for the 21st Century

Battery chemistries used in handportable radios
Nickel Cadmium
Until people became concerned at the environment, the most popular battery for hand held radios were of Nickel Cadmium (Ni-Cd) construction. Typically using a nickel oxide-hydroxide positive and cadmium negative plate connected by a potassium hydroxide electrolyte, the cell contents are extremely toxic. Nowadays they are considered a hazard in the factory whilst being assembled and are (in most countries) heavily regulated on disposal - landfill is no longer acceptable.
NiCd battery packs have a typical service life of around 800 to 1000 charge cycles but do suffer from memory effect. If repeatedly discharged partially before recharging, overly large crystals form within the electrolyte which cause voltage depression and high resistance. Repeated cycling between full discharge and full charge, 'conditioning' will often break these large crystals down however, allowing further use of the pack. These packs are well suited to radio use with an operating range of -30°C and +60°C and are usually undamaged by full discharge - indeed they are normally stored in an uncharged state.
Nickel Metal Hydride
During the 1990s the nickel-metal hydride (NiMH) battery became available. This is a variation of the Ni-Cd construction where the cadmium of the negative electrode is replaced by a complex rare earth alloy, typically a mixture of lanthanum, cerium, neodymium and praseodymium. Environmentally much preferred at it contains no toxic cadmium, these batteries also have a far higher energy density, typically 40% better than a Ni-Cd pack. NiMH batteries are much less susceptible to memory effect than Ni-Cd batteries, but are more temperature conscious, indeed use in 45°C conditions will reduce the cycle life by typically 60%. Cycle life is also typically halved by internal overheating during, or more commonly, at the end of charging if the wrong fast charger is used. The temperature change characteristics at the completion of charging are different to that of Ni-Cd batteries, so don't use the wrong charger - it must be designed for NiMH! In the absence of abuse, the working life in charge cycles and the working temperature range of the NiMH battery are very similar to the traditional Ni-Cd, although self-discharge is slightly greater.
Lithium Ion
By 1999 lithium-ion (Li-ion) batteries had made it out of the laboratory and on to the price list. A very different chemistry to that above, a typical Lithium-ion battery uses graphite for its negative electrode and lithium cobalt dioxide (LiCoO2) or a lithium manganese compound (LiMn2O4) as the positive. The electrolyte is usually based on a lithium salt in an organic solvent. The plate voltage varies with the exact chemistry but is around three times that of a Ni-Cd battery cell at around 4 volts fully charged. Lithium-Ion batteries have a much greater energy density than Ni-Cd batteries, nearly three times that of a Ni-Cd by weight and over twice by volume, although this advantage is reduced by the necessary support electronics incorporated in every battery pack. Service life is restricted severely by both over discharging and over charging: 10% under voltage will reduce life by typically 66% and 10% over voltage by 80%. Fortunately modern radios and chargers will check the voltage and close down when it gets out of the working window. Memory effect is not an issue, indeed frequent charging after partial discharging is actually beneficial to service life, using only 20% of a battery's charge then recharging, will extend the cycle life by five to six times the 500 full discharge/charge cycle life the batteries are usually rated at. Best usage is directly the opposite practice to that for the nickel based batteries. Whilst this makes for easier radio life - just work your shift, then recharge the battery regardless - service life is restricted by ageing, after two to three years (regardless of use) the internal resistance will have risen so much that the battery will no longer provide sufficient current to operate the radio: back to the dealer for a replacement, but hey, what a better signal when each set transmits at full power again! Storage requirements are different too, it is best to store Li-ion batteries close but above 0°C and at approximately half charge - storing them warm and fully charged (in a computer laptop plugged into the mains the battery will often exceed 40°C) will speed up ageing by nearly 20 fold.